Life Sciences
Clinical analytics built to stand up to regulatory review
Trusted in regulated clinical and submission workflows
• Deployed across enterprise R and Python environments in GxP contexts
• Used to standardize workflows from data preparation through final CSR outputs




Customer Impact
Cut trial reporting time without compromising reproducibility
Within 12 months, Roche reduced SDTM dataset creation time by half—while maintaining reproducible, submission-ready outputs.
• Automated pipelines for SDTM, ADaM, and TLG generation
• Controlled package and environment management to ensure consistent results
• Traceable workflows that support review, reuse, and regulatory confidence
Regulatory Compliance
Meet regulatory expectations with reproducible analytics
Posit supports workflows aligned with GxP, 21 CFR Part 11, and CDISC, helping teams produce analyses that can be rerun, reviewed, and explained during regulatory review.
• Lock package versions and analytical environments to ensure consistent, reproducible results
• Maintain traceability across code, data, and outputs to support audit and inspection review
• Centrally publish controlled, submission-ready artifacts aligned with CDISC standards

Open Source Value
Built for IT, QA, and compliance teams
• Deploy on-premises, in the cloud, or in hybrid architectures
• Integrate with SSO, LDAP, or Active Directory for centralized identity management
• Enforce role-based access controls with audit-ready activity logs
• Support secure and offline deployments, including air-gapped environments
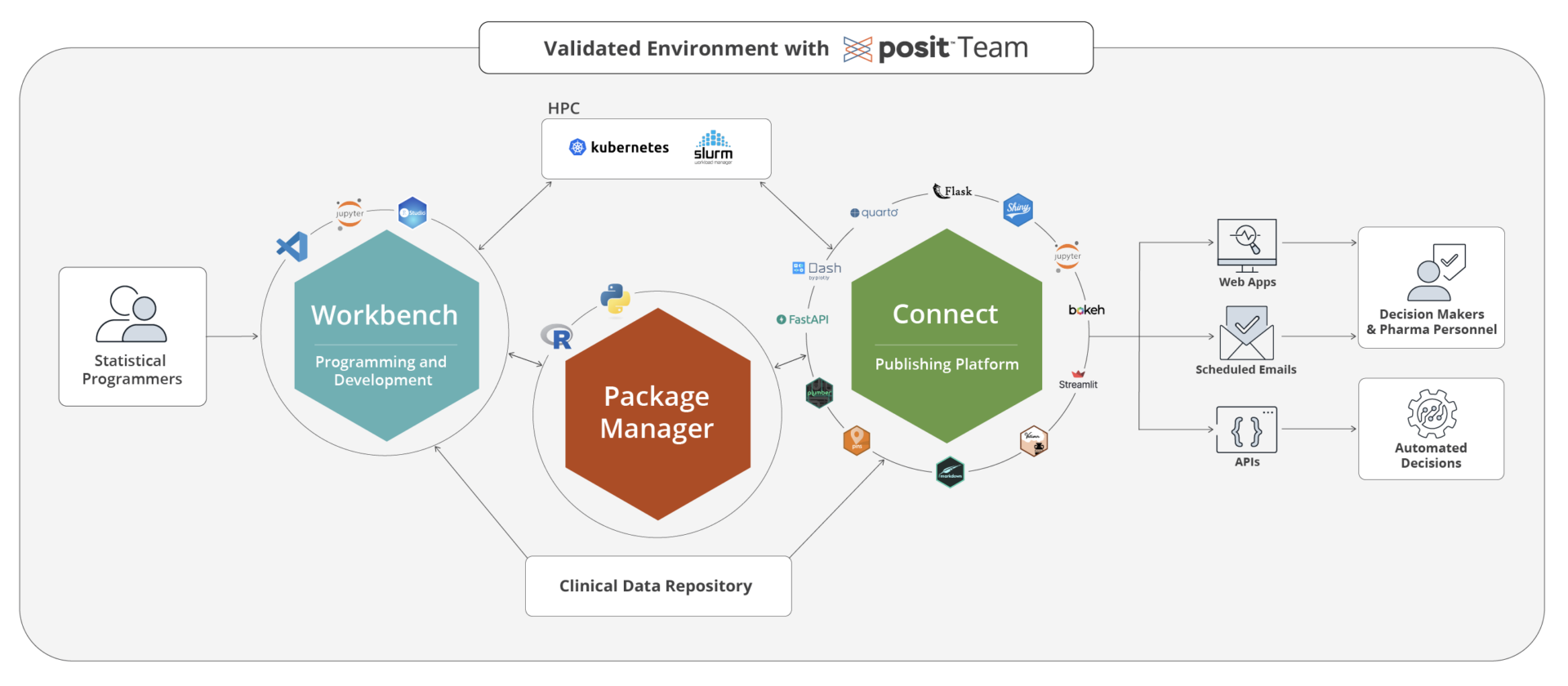
The Posit advantage
Standardize on R and Python without sacrificing governance or control. Posit supports the tools scientists already use—while giving organizations the structure needed for regulated work. • GSK committed to moving 50% of clinical code to R • Novo Nordisk delivered an R-based NDA submission in under two years
Posit supports and contributes to initiatives like Pharmaverse and the R Validation Hub, helping align open-source packages with clinical reporting and submission needs. This shared foundation reduces duplicated validation effort across sponsors and accelerates adoption of standardized, submission-ready workflows.
Posit is designed to support regulated analytics aligned with 21 CFR Part 11, GxP, and global regulatory expectations. Teams generate reproducible results with traceable provenance—without relying on manual controls or one-off validation.
Pharma Spotlight









