The next generation of open source SCEs
“The SCE is a multi-faceted platform primarily consistent of a highly GxP controlled environment that provides a foundation for documenting rigor in the analysis and reporting of clinical trial results” - PHUSE/SCE Council
Posit’s Professional Suite
Flexible professional products customizable to Pharma
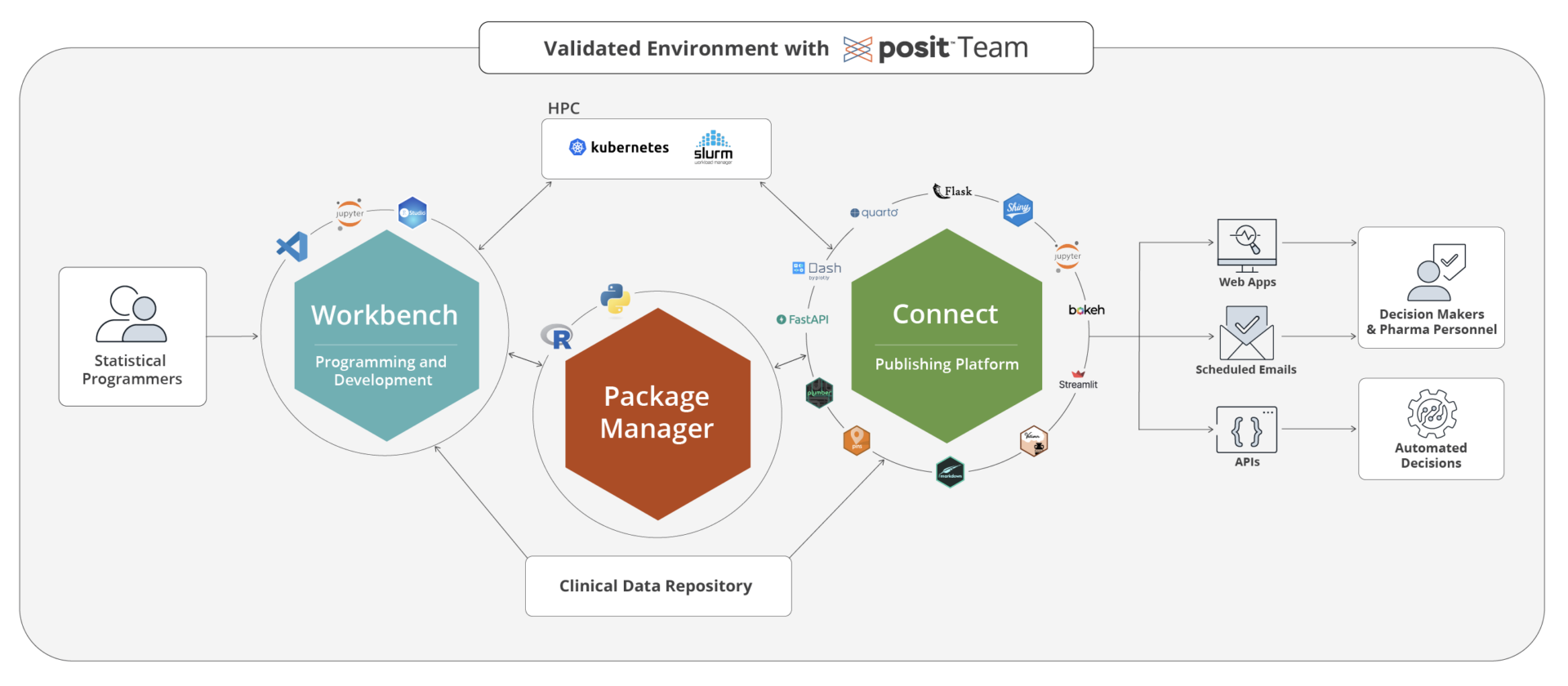
Build with Posit Workbench
Posit Workbench allows biostatisticians and statistical programmers to create insights using the IDE of their choice in order to inform go no-go decisions in clinical development. Everything lives on a centrally managed infrastructure that allows for multiple scaling options such as SLURM based High Performance Computing (HPC) or Kubernetes Clusters.
Share with Posit Connect
Share insights that shorten the feedback loops. Connect lets you share interactive visual dashboards that can give real-time data using Shiny and even Python for your clinical trial research – allowing decision-makers to dive into the data quicker at go/no-go checkpoints.
Host validated packages with Posit Package Manager
Posit Package Manager (PPM) allows you to locally host all the R and Python repositories your team uses and loves (e.g. CRAN, Bioconductor ,PyPi, …). In addition to that PPM can be used to offer all your tested and validated packages in a separate repository that then can linked to Posit Workbench and Posit Connect creating ease of mind for the users and ensuring a large degree of compliance when it comes to package usage.
Upskill talent with Posit Academy
Moving your workforce from proprietary languages to R or even Python can be made easier with a curriculum, mentor, and small group learning environments. Posit Academy throws you right into the types of open source work your team can expect to apply in their day-to-day workload.
Industry-Led Examples
The top 20 pharma companies in the world use Posit tools
Supporting the Validation Process
Ensuring quality verification in a statistical environment
End-user Tools for Reproducibility in your Environments
Maintain the modular SCE
Environments need to be reproducible, and this responsibility starts with IT qualification of the infrastructure and the operating system and software installation. But reproducibility does not stop on the IT side. Analyses run by biostatisticians need to be reproducible as well. Posit Workbench will help by directly offering or providing support for tools like renv, projects, version control, etc.
The Importance of Validation and Qualification
Do you need to validate your environment?
Absolutely. As a medical body, a regulatory agency needs to know that the code you ran a couple of days ago can be reproduced when asked. Organisations can integrate the setup Posit Team into their Computerized System Validation (CSV) process that will lead to a fully validated environment. CSV starts with user requirements gathering, includes IT Qualification of the infrastructure, operating system and software installation and will end with testing against the user requirements that will give the system the validation status.
Package Testing
Testing resources for packages
While everyone talks about package validation, the actual process can be more accurately described as package testing. Strictly speaking, you only can ever validate a workflow or business process but you never validate a software as such. However, when validating a workflow, it is imperative to ensure that the software used is producing correct results, for example and this can be achieved via package testing. There are many tools out there to help with automating those tests with some being listed below.
When it comes to packages, there are two categories to keep in mind: public packages and internal packages. Some Pharma specific packages are supported and maintained by communities within the Pharma space while others are regularly maintained and tested by repositories such as CRAN and others. Each repository has their own criteria and selection process to allow packages into that respective repository and as such the fact that a package is part of a given repository can provide insights into how trustworthy this package is.
Validation strategy and planning
While in principle a package would need to be tested for each of the functions it offers and for the full parameter space it allows, the testing effort very quickly can become a problem both in terms of time and resources. As a consequence, the typical approach used when testing (and later on validating) packages is risk-based validation.
The overall approach is documented in a validation strategy and validation plan. Both describe a consistent approach to package testing where clearly defined criteria are being used to infer the risk arising from a given package. This risk can be assessed by mixing various metrics (e.g. download count, open issues on github, number of reverse dependencies, …) For the risk assessment the Validation Hub has developed tools that simplify this assessment. Based on the calculated risk a specific testing strategy is informed (as defined in the validation plan). While undertaking such a rigorous risk assessment and testing can quickly become very time-consuming if done in-house, there are companies like Atorus that can help you here via their OpenVal product.
We play nicely with your existing tools





Documentation for packages and software created by Posit
Expanding the SCE
The other pieces to address in your environment
Security
When building out your statistical computing environment, consider how to add data protection to your existing processes and systems. Additionally, implementing access management to allow visibility to who needs it and when. Security is a top priority to keep data protected, auditable, and accessible to who needs it.
Infrastructure
Where your environment lives can impact what it can support. Think about computer power, resources, container orchestration, cloud security, and how to integrate current applications into future environments as you scale.
Data Management
Data is a huge part of the statistical computing environment and involves data connections, access to data, managing data warehouses or data lakes, storing locations, in addition to security. Our professional software can help support these drivers of data and there are partners that can help you integrate and access that data as needed.
Regulatory Compliance
Consider how to build your environment without feeling limited by compliance. You want to meet your compliance requirements by HIPAA and others while integrating your solutions into existing methods for data governance.